The house I live in was built at the turn of the century – the 18th century. And on really cold days I sometimes like to think of what firearms had walked in through the door in years past. More than 50 years before the birth of our nation, flintlock smoothbores put food on the table and protected the homestead. (Flintlocks. Wow, I get annoyed after loading five Glock mags.) But eventually the occupants here were blessed with centerfire guns, thanks in large part to the development of the primer.
So, let’s give some love to the tiny little primer that always gets taken for granted.
Physical Composition
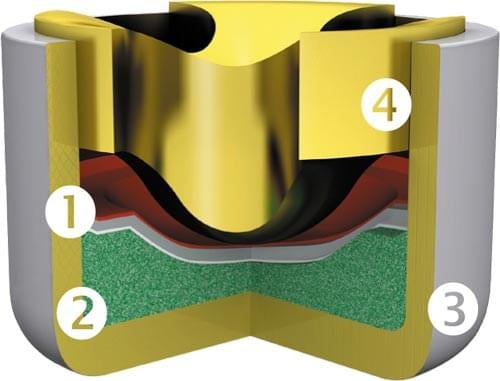
Credit: RWS Ammunition
- Cup – Holds the primer contents and is pressed/seated into the cartridge [#3 Above]
- Pressure/Friction sensitive explosive – See Chemical Composition below [#2 Above]
- Foil/Paper – Used as a separator [#1 Above]
- Anvil – Deformation of the cup by the firing pin compresses the friction-sensitive explosive against the anvil, starting detonation, forcing hot material into the gunpowder in the case. Depending on the type of priming system, the anvil is located in the cartridge, between the cartridge and the primer or directly in the primer itself. [#4 Above]
A common misconception is that the typical type of modern day primer is the Boxer System. In reality, most modern day priming systems are Anvil-based using a Boxer-type cartridge. The differences are very slight, but if we are going to talk technicals, let’s make sure to understand all the details.
Berdan primers use cartridges that have the anvil built into the cartridge. These are cheaper to manufacture, but make reloading difficult.
Boxer primers use cartridges have a detached anvil, separate from the cartridge itself. (Think cartridge, then anvil then primer).
Anvil primers contain the anvil inside the primer itself and use Boxer-type cartridges. Most modern civilian primers are of the Anvil type using a Boxer Cartridge.
Rimfire
A quick note on rimfire cartridges – the primer material is spun into the rim of each case using centrifugal force. The mashing of the rim edges together acts as a cap surface and anvil, starting the chemical reactions detailed below.
Chemical Composition
In the beginning, primers were comprised of highly reactive substances like Mercury Fulminate, Hg(CNO)2, as a primary explosive. It is highly sensitive to friction and shock.
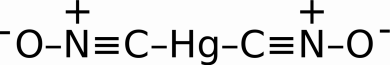
Mercury Fulminate
The problem with Mercury Fulminate, besides spraying vaporized Mercury everywhere, is that it degrades over time leaving the possibility for misfires or hangfires. Mercury Fulminate’s angry older brother, Silver Fulminate is even more unstable, often reacting (exploding) due to moisture and the friction caused by the pressure of its own weight.
Corrosive Primers
Transitioning to more modern times, Potassium Chlorate and Potassium Perchlorate were often used as primer oxidizers for lead thiocyanate and antimony trisulfide that would create incandescent particles that more effectively ignited smokeless powders.
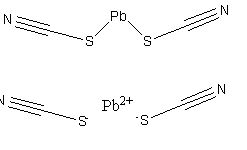
Lead Thiocyanate and its salt (bottom)
Potassium Chlorate is a compound containing potassium, chlorine and oxygen atoms, with the molecular formula KClO3. It acts as an oxidizer.

Potassium Chlorate
Potassium Perchloarate also is an oxidizer in the sense that it exothermically (generates heat) transfers oxygen to combustible materials, greatly increasing their rate of combustion relative to that in air.
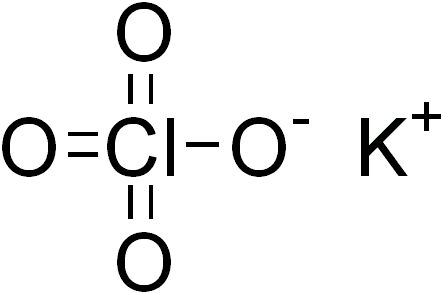
Potassium Perchlorate
The problem with these primer components is that they will create chloride salts that attract water and eventually cause corrosion. These primers are referred to as Corrosive Primers and still exist in older or some surplus military ammunition.
Non-Corrosive Primers
Modern, non-corrosive primers began development in the 1920’s and contain lead styphnate, barium nitrate, antimony trisulfide, powdered aluminum and tetrazene.
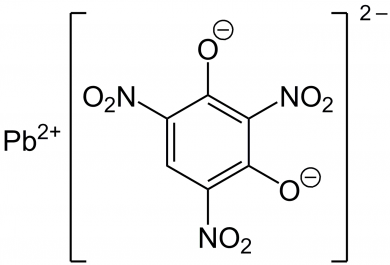
Lead Styphnate
Although these newer components are non-corrosive, they are still toxic to humans. So here’s your safety reminder – always wash your hands after a range session.
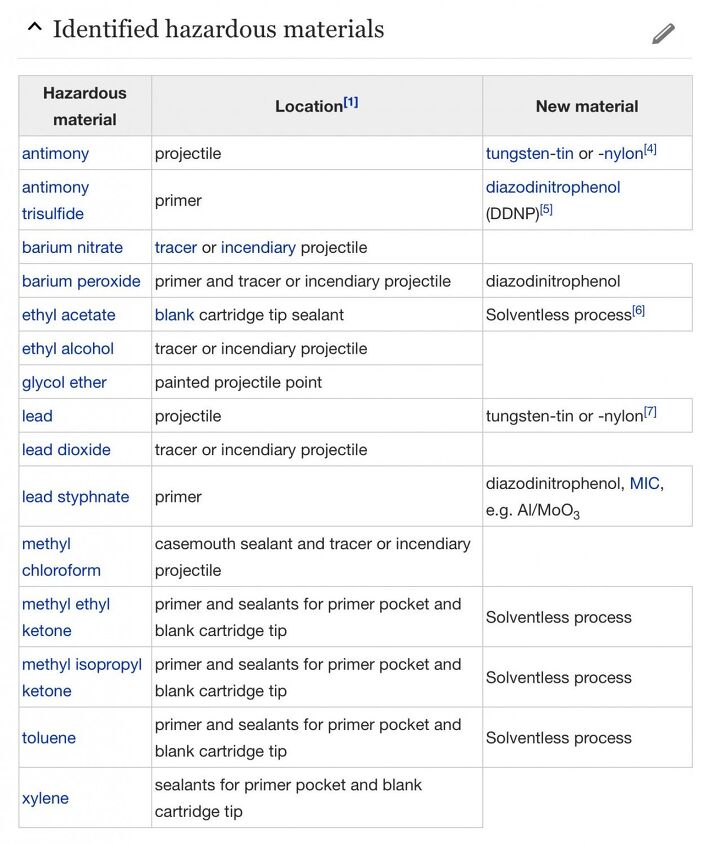
Green “Bullets” (cartridges/bullets/primers)
Another misconception about ammunition is that the lead-based projectile is the source of the majority of the toxicity found in the cartridge. And while it’s true that massive amounts of lead deposited at ranges can be problematic for the environment, a more immediate concern for shooters is the heavy metals in particulate or gaseous form, most of which come from the primer.
Many manufacturers have began to develop “green” bullets (cartridges really) that attempt to substitute toxic components for less toxic components. And while I applaud the effort, many of the new materials listed don’t exactly sound organic, even if they are free of heavy metals.
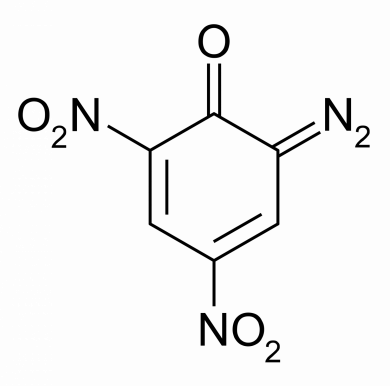
Diazodinitrophenol – Oxidizer in “green” primers.
In the end, each ammunition manufacturer is going to have a proprietary chemical composition for their primer mixtures, mostly focused on economy and reliability.
Why include the chemical structures of the above components? Because it’s important to notice the positive (+) and negative (-) charges keeping the molecule together. It’s these charges and attraction that makes the primer chemistry inherently unstable – each of these chemicals are dying to become more stable and given the chance (like a good hit, for example) will react, breaking bonds and releasing energy as an explosion.
Detonation:
The rest of the process is easy. Just make the firing pin hit the primer.

And that’s The More You Know – Primers.
Citations:
Hiram Berdan (US) 1824-1893 – https://en.m.wikipedia.org/wiki/Hiram_Berdan
Edward M. Boxer (UK) 1822-1898 – https://en.m.wikipedia.org/wiki/Edward_Mounier_Boxer
Centerfire Cartridges – https://en.m.wikipedia.org/wiki/Centerfire_ammunition
Mercury Fulminate – https://en.m.wikipedia.org/wiki/Mercury(II)_fulminate#
Potassium Perchlorate – https://en.m.wikipedia.org/wiki/Potassium_perchlorate#
Potassium Chlorate – https://en.m.wikipedia.org/wiki/Potassium_chlorate#
Lead Styphnate – https://en.m.wikipedia.org/wiki/Lead_styphnate#
Lead Thiocyanate – https://en.m.wikipedia.org/wiki/Lead(II)_thiocyanate#
RWS Ammunition Research and Development – https://rws-munition.de/en/rws-hunting-area/about-us/research-and-development/the-primer.html
 Your Privacy Choices
Your Privacy Choices
