Signaling the arrival of a surplus firearm from a time and world nearly forgotten, the sweet smell of the Cosmoline protective coating invokes a Pavlov-like reaction in even the most novice collector. Sure, the moment can be short lived as you decide on a removal method to get to the gun underneath. But as sticky and obnoxious as that amber colored mixture is, it’s hard not to appreciate its amazing corrosion resistance properties.
But what exactly is Cosmoline? What makes it such a great long-term protective coating? Should I use it on my guns? Do they even make Cosmoline anymore?
Standby. I’ll see what I can do…

Actually, that is just tree sap.
Here’s my standard disclosure: It’s been almost 20 years since I’ve used chemistry in my professional life. Undoubtedly, a chemical engineer or petroleum scientist is going to come along and say “that’s not exactly true”. You’re right; just consider this a “Cosmoline chemistry for trigger-pullers” write up.

In it’s most popular form, Cosmoline has been around for nearly 200 years. However, if you believe unsourced Wikipedia statements, chemically similar substances have been found being used as a preservative in ancient Egyptian pyramid tombs.
For better or worse, the ‘original’ Cosmoline product doesn’t exist anymore. The current exclusive distributor markets a product called “Original Cosmoline” or Rust Veto 342 which is as close to the real thing available. However, it is actually more of a liquid than a gel like the OG coating.
From the Schafco Information Page:
Cosmoline was developed by Houghton International in the late 1800’s as a pharmaceutical product. The original Cosmoline was basically an ointment and was used for many different cosmetic and medical purposes. It was kept in homes to disinfect wounds and was used by veterinarians to treat cuts, abrasions, bruises and sprains. Cosmoline could even be found on the farms where it was used to relieve swelling in cow’s udders.
As industry changed so did Cosmoline. New formulations of Cosmoline were developed to meet the ever growing need. Cosmoline products were available in ranges from a light type fluid to a thick, heavy grease meant for long term protection. Cosmoline’s versatility was unparalleled.
Cosmoline became an everyday name when it received a government specification as a rust preventive and began being used by the military to protect it’s equipment from rust and corrosion. Cosmoline could be found on military equipment in the Spanish-American War, World War I, World War II, the Korean conflict and Vietnam.
In 1958 Schafco Packaging began packaging Cosmoline products into aerosol versions for Houghton International and in 2004 Schafco became the exclusive distributor for the Cosmoline Aerosol line of products.

Rust Veto 342 i.e. “Original Cosmoline” i.e. “Coke Classic”
That’s great and all, but what actually is Cosmoline?
From the Cosmoline Wikipedia Page:
Chemically, cosmoline is a homogeneous mixture of oily and waxy long-chain, non-polar hydrocarbons. It is always brown in color, but can differ in viscosity and shear strength. Cosmoline melts at 113–125 °F (45–52 °C) and has a flash point of 365 °F (185 °C).
Of course, oily and waxy long-chain non-polar hydrocarbons! Should have known…
Let’s take a look at the chemical composition of wax.
From the Parafin Wax Wikipedia Page:
Hentriacontane, also called untriacontane, is a solid, long-chain alkane hydrocarbon with the structural formula CH3(CH2)29CH3.
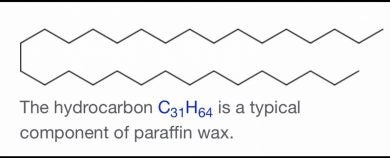
Long-chain hydrocarbons like wax are exactly that – long chains of bonded carbon and hydrogen. In the image above, the carbon atoms are represented at the points in the chain. The hydrogen atoms are taken for granted as two bonds off each of the carbon. This coincides with the more than two to one ratio of hydrogen to carbon atoms – 31 carbons and 64 hydrogens (the carbons at the chain ends have three hydrogen bonds).
Who cares? What does this have to do with Cosmoline being awesome?
Long chain hydrocarbons are hydrophobic, meaning they “fear” (repel) water. But their non-polar characteristics give them an added anti corrosive benefit.
Non-Polar Hydrocarbons From ‘What Is Chemistry’:
Because hydrocarbons consist only C-H bonds, which are non-polar. When you look at the table of electronegativity (Pauling scale), you see, that the difference between the electronegativities of carbon and hydrogen is much smaller than that of nitrogen and hydrogen. That is why N-H bonds are considered polar, and C-H bonds are not.
Without getting super-technical, the Pauling Scale gives a electromagnetic charge value to each element. Since the long chain, non-polar hydrocarbons are comprised of only Carbon and Hydrogen , there is no excessive charge differential like “head and tail” polar molecules have. The head of a polar hydrocarbon is a bond with another atom like nitrogen.
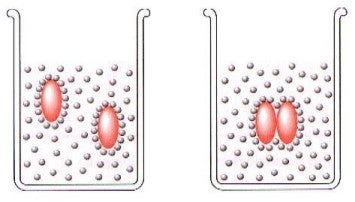
Non-Polar solution, Left. In hydrophobic reaction , Right.
Usually, non polar compounds are not water soluble because they contain neither ionic groups nor polar functional groups that can interact favorably with water molecules. Those compounds are called “hydrophobic” (water fearing), because they escape the contact with water. Hydrocarbons, constituted by carbon and hydrogen only, are examples of such a kind of compounds. When non polar molecules enter aqueous medium, some water-water hydrogen bonds are to be broken to create a cavity for the solute molecule. Each solute molecule is entrapped in a cage, ice-like, structure formed by highly ordered water molecules, held together by hydrogen bonds. The formed complex, with the non polar molecule in the centre encircled by the cage of water molecules, is called “hydrate”.
Water molecules in the cage around the non polar molecule are more ordered than in pure water. In the presence of many hydrocarbon molecules, the same number of ordered cages of water molecules should form, with the consequent large increase in the order of the system, a process naturally unfavorable. To minimize the increase in the order of the system, hydrocarbon molecules, each one encircled by its own cage of water molecules, associate together (hydrophobic interaction). If, for sake of simplicity, only two non polar molecules in aqueous solution are considered, hydrophobic interaction causes the non polar molecules to come together into a single cavity to reduce the unfavorable interaction with water.
To put it simply, polar hydrophobic substances create the opportunity to allow water in and interact with the iron (the gun? and iron alloys to corrode (rust). Non-polar hydrocarbons pull water in out of solution to be encapsulated and away from the iron. No rust!
But what else is in Cosmoline?
From the (current) Cosmoline Manufacterer SafeTy data sheet (MSDS):
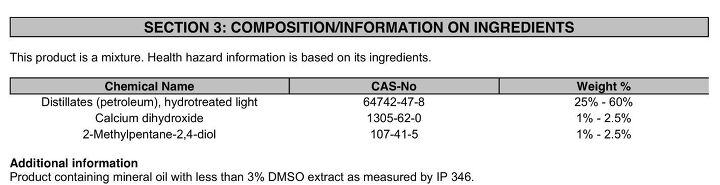
Calcium dihydroxide is most likely added in small amounts to clarify the solution and to aid in the water encapsulation process.
2-Methylpentane-2,4-diol is used to lower surface tensions between two liquids (oils/waxes and water, for example):
2-Methyl-2,4-pentanediol exhibits both surfactant and emulsion-stabilizing properties. Its relatively high viscosity and low volatility are advantageous in coatings, cleansers, cosmetics, solvents, and hydraulic fluids.
One major difference between today’s Cosmoline and the original formulations we know and love is that it has a lower melting point. This means removal of Cosmoline with moderate heat is easier, however storage of items in higher heat environments is more challenging.

Is Cosmoline still available and does it still have a place in firearm storage?
Yes and yes. Several formulations are available for retail sale at CosmolineDirect. As far as long-term corrosion protection, follow the preparation and application procedures and your EOTWAWKI guns will be just fine.
That is, unless earth is attacked by aliens who excrete high temperature, high pressure solvents or detergents. In that case our Nagants, and the world as we know it, are toast.
Cosmoline Chemistry – That’s ‘The More You Know’

 Your Privacy Choices
Your Privacy Choices
