I like cleaning guns. One, you usually have to be surrounded by guns to clean them. And two, for the most part there is a direct relationship between the time you invest and the results on the other end. However, I concede that I might be in the minority when it comes to breaking out the Ballistol and patches. And even more so when it comes to cleaning rimfire silencers.
Modern rimfire and pistol caliber suppressors are designed to be disassembled and cleaned regularly. Even so, rimfire and cast lead bullet loadings can make a silencer internals pretty messy. And while there are a variety of techniques to cleaning baffles, one technique regularly discussed on internet forums is simply called “The Dip”.
WARNING: I like ‘do it yourself’ projects like most gun owners, however, I cannot recommend making or using “The Dip” to clean suppressors. It is a highly toxic solution that will poison or kill you if handled incorrectly.
“The Dip” is created by mixing a 50/50 mixture of White Vinegar (Acetic Acid) with Hydrogen Peroxide. The resulting mixture can then be used to dissolve the lead that is caked on stainless steel baffles. Dropping lead-coated silencer parts into the reactive solution slowly creates Lead Acetate, which is basically dissolved lead in solution.
From the Lead Acetate Wikipedia page:
Lead acetate can be made by boiling elemental lead in acetic acid and hydrogen peroxide. This method of using acetic acid and hydrogen peroxide will also work with lead carbonate or lead oxide.
(Not only is a bad idea to make this solution, it’s doubly bad to boil it.)
Pb(s) + H2O2 + 2 H+(aq) → Pb2+(aq) + 2 H2O(l)
Pb2+ + 2 CH3COO−(aq) → Pb(CH3COO)2
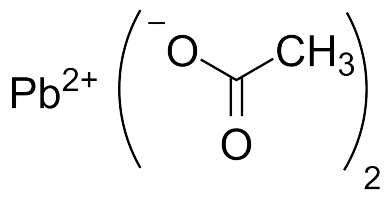
Lead Acetate
Whereas common (elemental) lead poisoning most commonly occurs by ingesting and absorption of lead in the gastrointestinal system, poisoning via Lead Acetate can occur with simple contact with the dermis (skin). Lead Acetate must be treated as a hazardous substance and dealt with appropriately.
A current thread on Silencer Talk documents the use of “The Dip” on a rimfire suppressor with approximately 400 rounds clocked since the last cleaning.
Bowen1911: I decided to snap some pics as i cleaned out my silencer last night. I put 333 winchester bulk pack and 100 remington subsonics through it since the last cleaning

Bowen1911

Bowen1911

Bowen1911

Bowen1911

Bowen1911

Bowen1911
As you can see, “The Dip” is a very effective cleaning solution.
A couple of things:
- Please do not make or use “The Dip”.
- If you have any of this solution around your shop, treat it as a hazardous material: wear gloves and other protective gear.
- Disposing of “The Dip” means taking it to a certified hazardous waste collection company/department.
- It has been a long time since organic chemistry, but there are ways to neutralize the solution as well precipitate out the lead (salts) from “The Dip” – (I’m being vague here because I don’t want to advocate using “The Dip” for cleaning silencers).
- “The Dip” will damage aluminum.
Next week we will talk about two other ways to clean suppressors: “Soda Blasting” and Tumbling. Both are much safer than creating Lead Acetate.
 Your Privacy Choices
Your Privacy Choices
